Custom Implant Devices
The Centre for Implant Technology and Retrieval Analysis (CITRA) provides patient specific solutions in cases of complex trauma or skeletal deficiency that cannot be addressed using the available commercial device options.
Implant device, procedural and instrumentation solutions require a clinical referral and are based on imaging data and computer assisted design, solid modelling, rapid prototyping and the support of Advanced Manufacturing.
Service support is underpinned by research and development activity in the areas of biomaterials and tissue engineering conducted in alliance with Cell and Tissue Therapies WA, co-located at Royal Perth Hospital.
Innovation relies on the application of new techniques and thinking to solve a defined problem. In the case of a hospital based, clinical support service such as CITRA, these problems are defined by problems experienced in providing or improving the patient service. Despite having access to an extensive inventory of implant devices, the orthopaedic surgeon is still confronted by unique conditions that require customised solutions.
CITRA was one of the first providers in Australia of custom medical devices to meet this need, with the first implanted device provided to a patient in the 1980s. Advances such as improved imaging techniques, medical modelling and the introduction of improved manufacturing techniques such as 3D printing have contributed to clinical success and improved productivity.
Clinical Imaging
 Since the 1980s, computed tomography (CT) and magnetic resonance imaging (MRI) techniques have been used as source data from which custom medical devices are designed.
Since the 1980s, computed tomography (CT) and magnetic resonance imaging (MRI) techniques have been used as source data from which custom medical devices are designed.
Surface scanning devices are used to scan the patient’s external anatomy to aid in the design of custom devices such as ears, noses or ensuring cosmesis in reconstruction cases.
Medical Modelling
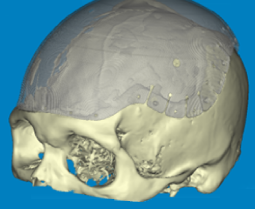 Advanced software is required to apply digital images for use in custom implant design. The design of a patient specific implant requires that the model is accurate and that the available data is moderated by clinical judgement and experience.
Advanced software is required to apply digital images for use in custom implant design. The design of a patient specific implant requires that the model is accurate and that the available data is moderated by clinical judgement and experience.
A clear understanding of the individual’s causative pathology and/or the likely effects of surgical implantation is essential in qualifying the anatomical model as one that is relevant to the patient condition; the medical model.
Advanced Manufacturing
 The recent decades have seen a transformation in manufacturing from that of the traditional subtractive machining (e.g. lathe and milling technologies) to that of additive manufacturing (commonly known as 3D printing). Additive manufacture is particularly suited to custom implant development that is based on complex 3D data with the potential for it being produced locally and/or remotely. The evolving development of metal substrates has improved the prospect of direct manufacture of implant devices as distinct from rapid prototyping and the use of conventional machining and fabrication techniques. To facilitate the transition to advanced manufacturing, CITRA uses a combination of in-house and out-sourced services.
The recent decades have seen a transformation in manufacturing from that of the traditional subtractive machining (e.g. lathe and milling technologies) to that of additive manufacturing (commonly known as 3D printing). Additive manufacture is particularly suited to custom implant development that is based on complex 3D data with the potential for it being produced locally and/or remotely. The evolving development of metal substrates has improved the prospect of direct manufacture of implant devices as distinct from rapid prototyping and the use of conventional machining and fabrication techniques. To facilitate the transition to advanced manufacturing, CITRA uses a combination of in-house and out-sourced services.
Bioengineering at Royal Perth Hospital dates back to 1969 when the first engineer was employed by the hospital to provide specialist medical engineering services directly to clinicians. The early emphasis was on the areas of orthopaedics, spinal injuries and rehabilitation. Since that time the service, now known as CITRA, has developed into a world class facility providing a comprehensive range of bioengineering services to hospitals throughout Western Australia. The key focus for CITRA is in the areas of implant technology, retrieval analysis and in the emerging area of tissue engineering. More than 10,000 devices removed as a routine or due to failure/revision have been subject to investigation and over 1,000 patients during the last 20 years have benefited from specialised custom medical devices – a truly life improving service to Western Australians.
Visit the history and achievements page for more information about CITRA and custom implant devices.
One key to providing quality services in support of the clinical process is to remain aware of contemporary practices and advances in technology and, where appropriate, to apply them directly to the delivery of patient care and to improve the standard of services that support that care. The Bioengineering Division are early adopters of new and innovative technologies that are used routinely in support of service provision.
Check out the Using Contemporary Technology page to learn more about the new and innovative technologies being adopted by CITRA.
Tissue engineering and biofabrication encompasses the production of replacement tissues and organs to address the patient need. It is based on additive manufacturing and uses techniques such as 3D printing to produce scaffolds upon which living cells can be placed, or bioprinting where a combination of a scaffold, living cells, gels fibres or pharmaceuticals can be printed to form live tissue.
Learn more about tissue engineering and biofabrication.
An Advanced Biomedical Research and Manufacturing Program
Traditional surgical treatment has long been based on ‘off the shelf’ solutions, whereby a limited range of standard sizes and shapes of devices necessitate surgically adapting the patient’s anatomy to the constraints of the implant. In contrast, personalised medicine, in which an implant or device is bespoke has the potential to revolutionise treatment. In order to realise this new surgical paradigm, tissue engineering, stem cell therapy, 3D printing and biofabrication represent some of the most exciting technologies that can be used individually or in combination to produce patient matched devices.
Find out more information about this unique biomedical research and manufacturing program.

